Discover a new era of automated T-SPOT™.TB testing.
The TB test you want. The automation you need.
See the Auto-Pure 2400 in action with the T-SPOT.TB test.
T-SPOT.TB enables accurate TB testing1 and when paired with our automation solutions you get efficient laboratory workflows without compromising on clinical performance.2
This powerful combination not only simplifies T-SPOT.TB testing but also provides confidence in the result.
- ✓ Efficient workflow, more time for you.
- ✓ Easy to use, easy to get right.
- ✓ Access high-quality testing, be confident in the result.
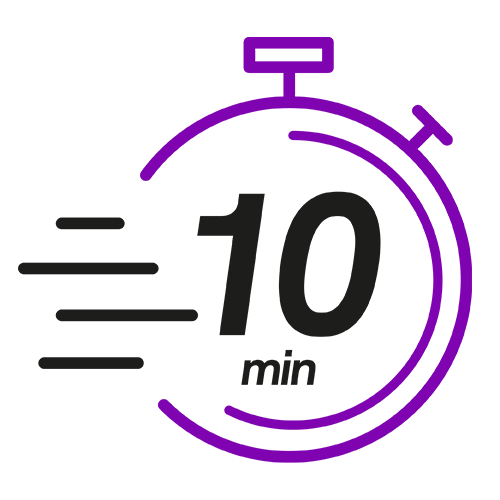
Less than 10 mins hands-on time once the Auto-Pure 2400 workflow has commenced*
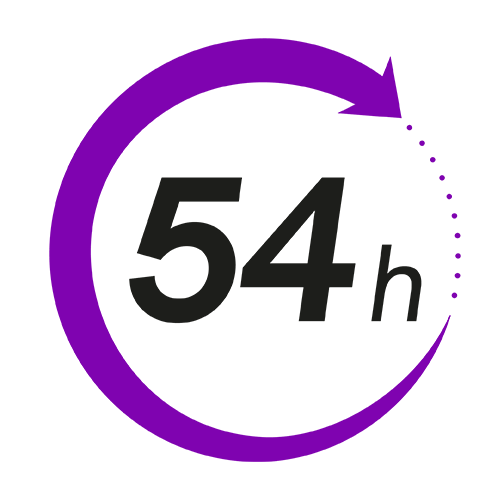
54-hour sample stability2

Only one primary tube needed*
Accurate TB testing with the T-SPOT technology.
The T-SPOT.TB test has three crucial steps that have recently been acknowledged by the WHO for ensuring reproducibility and mitigating the impact of pre-analytical variables.7 These steps include isolating, washing, and counting the PBMCs before the test is performed. Shifting from traditional whole blood sample testing, the T-SPOT.TB test provides precision and reliability, allowing more control in your TB infection testing.
The RIGHT cells
Selecting the right cells by isolating them
The desired cell population (PBMCs) are extracted from whole blood:
- PBMCs include lymphocytes (T cells) which are crucial for the immune response.
- CD4 and CD8 T cells are essential for detecting and managing TB through their roles in orchestrating and executing the immune response.
Key benefit: Specifically measure the release of IFN-gamma from T cells in response to TB-specific antigens
ONLY the right cells
Having only the right cells by washing them
The washing step enables the removal of potential interfering substances from whole blood, which could interfere with the cellular immune response and impact the performance of the assay.4,5
Examples of interfering substances:
- Drugs e.g., non-steroidal DMARDS, corticosteroids, tricyclic anti-depressants
- Endogenous IFN-gamma
Key benefit: Effective test in patients receiving treatment
ENOUGH of the right cells
Using enough of the right cells by counting them
Counting cells ensures the same number of cells are used per test facilitating correction for variations in patient cell counts.3
This is particularly important for patients who may have:
- Too many or too few cells
- Low cell numbers, frequent with autoimmune diseases such as chronic inflammatory bowel diseases or autoimmune hepatitis.6
Key benefit: A standard number of cells per test promotes reproducibility

High sensitivity and specificity providing accurate results.1

Low indeterminate results, few repeat tests7
Maintains performance in the immunosuppressed4,8
* Mid run user intervention with Auto-Pure 2400 workflow with Cellaca MX
* In immunosuppressed individuals two tubes may be requested
- Oxford Immunotec. T-SPOT.TB Package Insert PI-TB-IVD-UK-V6. Abingdon, UK. September 2024. Oxford Immunotec. T-SPOT.TB Package Insert PI-TB-US v14. Abingdon, UK. March 2025.
- T-Cell Select Package Insert PI-TS-IVD-UK-V9 Abingdon, UK. September 2024. T-Cell Select Package Insert PI-TS-IVD-US v6. Abingdon, UK. March 2025.
- World Health Organization. WHO operational handbook on tuberculosis. October 1, 2022 (https://www.who.int/publications/i/item/9789240058347)
- Bèlard, et al. Prednisolone treatment affects the performance of the QuantiFERON gold in-tube test and the tuberculin skin test in patients with autoimmune disorders screened for latent tuberculosis infection. Inflammatory Bowel Diseases, Volume 17, Issue 11, 1 November 2011, Pages 2340–2349.
- Wong SH, Gao Q, Tsoi KK, Wu WK, Tam LS, Lee N, Chan FK, Wu JC, Sung JJ, Ng SC. Effect of immunosuppressive therapy on interferon γ release assay for latent tuberculosis screening in patients with autoimmune diseases: a systematic review and meta-analysis. Thorax. 2016 Jan
- Koetz K at al. T cell homeostasis in patients with rheumatoid arthritis. Proc Natl Acad Sci U S A. 2000 Aug 1;97(16):9203-8. doi: 10.1073/pnas.97.16.9203. PMID: 10922071; PMCID: PMC16846
- Rego K, et al. Utility of the T-SPOT®.TB test’s borderline category to increase test resolution for results around the cut-off point. Tuberculosis. 2018;108:178-185.doi:10.1016/j.tube.2017.12.005.
Products may not be licensed in accordance with the laws in all countries. Please check with your local representative for availability.
LPG-MPN906-01
For In Vitro Diagnostic Use
Revvity is a trademark of Revvity, Inc. All other trademarks are the property of their respective owners.